JVIR INSTRUCTIONS FOR AUTHORS
(April 9, 2024)
Table of Contents
About JVIR and Instructions for Authors
Legal Considerations
Exclusive Submission Policy
Submission Declaration and Verification
Preprint Servers
Copyright
Ethics
Ethics in Publishing
Studies in Humans
Studies in Animals
Authorship
The Use of Artificial Intelligence (AI)
Sponsorship and Funding: Sources and Roles
Conflicts of Interest
Redundant (Duplicate) Publications, Piracy, and Plagiarism
Reporting Standards
SIR Reporting Standards
Standards by Study Type
CONSORT
STROBE
PRISMA
ARRIVE
Statistical Methods and Standards
Open Access
Funding Body Agreements and Policies
Authors for Whom English Is Not the Primary Language
Manuscript Preparation
Document Technical Specifications
Blinding of Manuscript
Key Words
Types of Submissions
Clinical Study and Laboratory Investigation
Brief Report

Letter to the Editor (including Case Reports)
Evidence-Based and Narrative Reviews
Research in Translation
Study Protocol Design
Lessons in IR (Morbidity & Mortality)
Extreme IR
Images in IR
In Memoriam
Editorial, Commentary, IR History, Book Review, Perspective
Supplements, Conference Proceedings, Meeting Abstracts
SIR and SIR Foundation Documents
Online-Only Publication
Supplementary Materials
Data Repository
Manuscript Submission Process
General Information
New Submissions
Revisions
File Names
Technical Specifications for Tables and Figures
Tables
Figures (Images)
Color Figure Policy
Technical Specifications for Radiographs, Photographs, Scanned Images, Halftones
Technical Specifications for Line Art, Diagrams, Drawings, Graphs
Size, Resolution, Labels, Arrows
Video Figures
Visual Abstracts
Proofs

ABOUT JVIR AND INSTRUCTIONS FOR AUTHORS
The Journal of Vascular and Interventional Radiology (JVIR) is devoted to the timely publication of
peer-reviewed clinical and laboratory studies in the field of vascular and interventional radiology. JVIR
is the official journal of the Society of Interventional Radiology (SIR). Statements made in published
articles are the responsibility of the authors and not that of JVIR or SIR.
These instructions follow the Uniform Requirements for Manuscripts Submitted to Biomedical Journals
(N Engl J Med 1997; 336:309 or see www.icmje.org/about-icmje/faqs/icmje-recommendations
).
Manuscripts should be prepared according to the American Medical Association Manual of Style, 11
th
edition (
www.amamanualofstyle.com). Once accepted, manuscripts are copy edited to conform to JVIR’s
standards and style. Accepted manuscripts become the property of JVIR and may not be published in
whole or in part without the express written permission of the author(s) or SIR (see section below, Rights
and Permissions).
All manuscripts must be submitted online at www.editorialmanager.com/jvir
. As part of the submission
process, authors will be required to complete a certificate of exclusive submission and financial disclosure
forms that will compose the conflict of interest disclosure statement to be included as a footnote on the
first page of the article. For officers or employees of the U.S. government, JVIR recognizes that works
prepared as part of official government duties are in the public domain, but government authors must still
complete the relevant forms.
Questions related to submissions or reviews should be addressed to the JVIR Publications Coordinators
at jvir@sirweb.org
. Questions related to editorial issues should be addressed to the SIR Director of
Publications Brian Haefs at bhaefs@sirweb.org or Managing Editor Ana Lewis at alewis@sirweb.org.
LEGAL CONSIDERATIONS
EXCLUSIVE SUBMISSION POLICY
JVIR adheres to the best publishing practice guidelines, as outlined by the SIR Code of Ethics
(principle 8) and the Committee on Publishing Ethics. Please visit
www.sirweb.org/about-
sir/governance/policies/ and www.publicationethics.org for details.
JVIR encourages maximum disclosure about similar material already published or submitted for
publication elsewhere at the time of submission to JVIR. This principle applies to both original
and review articles. Manuscripts will only be reviewed and accepted with the understanding that
they are contributed solely to JVIR. Authors must be certain that no manuscript on the same or
similar material has been, or will be, submitted to another journal by themselves, their co-authors,
or others at their institution prior to their work appearing in JVIR without notifying the editor.
The submission by authors of similar material to advertising, broadcast, or electronic media must
be indicated at the time of manuscript submission to the JVIR Editorial Manager system.
SUBMISSION DECLARATION AND VERIFICATION
Submission of an article implies that the work described has not been published
previously (except in the form of an abstract or as part of a published lecture or academic
thesis or as an electronic preprint; see
www.elsevier.com/editors-update/story/publishing-
ethics/clarification-of-our-policy-on-prior-publication), that it is not under consideration

for publication elsewhere, that its publication is approved by all authors and tacitly or
explicitly by the responsible authorities where the work was carried out, and that, if
accepted, it will not be published elsewhere in the same form, in English or in any other
language, including electronically, without the written consent of the copyright holder.
To verify originality, every article will be checked by the originality detection service
CrossCheck: www.elsevier.com/editors/plagdetect
. JVIR uses CrossCheck on every
submission. CrossCheck is a subscription service, but authors are encouraged to use any
available duplication checking software to check their own manuscript prior to
submission.
At the time of manuscript submission, authors must sign a Certificate of Exclusive
Submission to attest that no manuscript on the same or similar material has been, or will
be, submitted to another journal by themselves, their co-authors, or others at their
institution prior to its appearance in JVIR.
PREPRINT SERVERS
Posting of a manuscript on a preprint server prior to submission is not necessarily
considered to be prior or duplicate publication. However, JVIR editors consider novelty
when making manuscript decisions, and if a manuscript receives substantial publicity
before or during the peer-review process, suitability for publication may be
compromised. JVIR adheres to a double-blind peer review process, and discoverability of
the work on a preprint server may jeopardize the confidential review process.
1) Upon first submission to JVIR, the authors must inform the journal in the cover letter
that the manuscript has been posted to a preprint server and provide the name of the
server, the copyright license under which the manuscript is posted, and any associated
accession numbers or digital object identifiers (DOIs).
2) Only original author-prepared files may be posted. Versions of a manuscript that have
been prepared by a publisher or altered as a result of the peer-review process may not be
posted.
3) The authors must retain rights to copyright the work after posting on the preprint
server and, upon acceptance to JVIR, must be able to transfer the copyright to SIR.
4) A preprint DOI must be assigned to the posted preprint. Upon acceptance to JVIR, a
new DOI will be assigned to the article by JVIR. Once the article has been published in
its final form on the JVIR website, it is the author’s responsibility to update the preprint
server with an addendum stating that the peer-reviewed and edited version is now
published, with a link to the article on JVIR’s website.
COPYRIGHT
Upon acceptance of an article, authors will be asked to complete a 'Journal Publishing Agreement'
(see www.elsevier.com/about/policies/copyright
). An e-mail will be sent to the corresponding
author confirming receipt of the manuscript together with a 'Journal Publishing Agreement' form
or a link to the online version of this agreement.

Subscribers may reproduce tables of contents or prepare lists of articles including abstracts for
internal circulation within their institutions. Permission
of the Publisher is required for resale or
distribution outside the institution and for all other derivative works, including compilations and
translations. If excerpts from other copyrighted works are included, the author(s) must obtain
written permission from the copyright owners and credit the source(s) in the article. Elsevier has
preprinted forms (www.elsevier.com/__data/assets/word_doc/0007/98656/Permi
ssion-Request-
Form.docx) for use by authors in these cases. Authors (or authors’ employer or institution) have
certain rights to reuse the work: www.elsevier.com/about/policies/copyright.
ETHICS
ETHICS IN PUBLISHING
Please see our information pages on Ethics in publishing
(www.elsevier.com/about/policies/publishing-ethics
) and Ethical guidelines for journal
publication (www.elsevier.com/authors/journal-authors/policies-and-ethics)
STUDIES IN HUMANS
If the work involves the use of human subjects, the author must ensure that the work described
has been carried out in accordance with The Code of Ethics of the World Medical Association
(Declaration of Helsinki) (
www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-
principles-for-medical-research-involving-human-subjects) for experiments involving humans.
The manuscript should be in line with the Recommendations for the Conduct, Reporting, Editing
and Publication of Scholarly Work in Medical Journals (
www.icmje.org/recommendations) and
aim for the inclusion of representative human populations (sex, age and ethnicity) as per those
recommendations. The reporting of “sex,” “gender,” “race,”, and “ethnicity” should conform with
the SAGER guidelines (doi:10.1186/s41073-016-0007-6) and the updated AMA guidelines
(doi:10.1001/jama.2021.13304).
If an Institutional Review Board (IRB) exists at the institution(s) in which any study involving
human subjects is conducted, the investigators must obtain prior IRB approval. This requirement
applies to prospective and retrospective studies (including technical notes and case reports) that
involve any direct interaction with patients or evaluation or review of protected health
information (e.g., imaging studies or medical record reviews). Authors are required to specify the
IRB institution and approval protocol number on the title page, but need only specify IRB
approval in the text of the submitted manuscript. See Valji K. IRB Approval—Who Needs It?,
www.jvir.org/article/S1051-0443(07)61714-X/fulltext
.
If the IRB at the participating institution does not require approval for the type of research being
performed, a statement to this effect must be included in the manuscript. If no IRB existed at the
time the study was initiated, the authors must include a statement in the manuscript to this effect,
as well as a second statement that the principles of the Declaration of Helsinki were followed. If a
manuscript reports on the emergent use of a material or device not approved by the U.S. Food and
Drug Administration or accepted as standard practice, the authors must state that they obtained
informed consent from the patient (when feasible) and reported the case to the local IRB within
one week of the event. This procedure is only valid for a single patient.
The privacy rights of human subjects must always be observed. It is the author’s responsibility to
ensure that patient anonymity is carefully protected. Authors from U.S. institutions must comply
with all regulations of the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Unless authors have written permission from the patient (or, where applicable, the next of kin),
the personal details and protected health information (PHI) of any patient included in any part of
the article and in any supplementary materials (including all illustrations and videos) must be
removed before submission.
Studies on patients or volunteers require informed consent, which should be documented in the
manuscript. Written consents for participation in research and consents for publication of
personal details must be retained by the author but copies should not be provided to the journal.
Only if specifically requested by the journal in exceptional circumstances (for example if a legal
issue arises), the author must provide copies of the consents or evidence that such consents have
been obtained. For more information, please review the Elsevier Policy on the Use of Images or
Personal Information of Patients or other Individuals (
www.elsevier.com/about/policies/patient-
consent).
For prospective trials, randomized or not, authors should adhere to the recommendations of the
ICMJE to register the trial on clinicaltrials.gov
or the WHO International Clinical Trials Registry
Platform (www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-
registration.html). The registration number must be provided on the title page, but should not be
specified in the text to protect the confidentiality of the double-blinded review.
STUDIES IN ANIMALS
All animal experiments should be carried out in accordance with the U.K. Animals (Scientific
Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal
experiments (www.ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm
), or the
National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications
No. 8023, revised 1978) and the authors should clearly indicate in the manuscript that such
guidelines have been followed. The sex of animals must be indicated, and where appropriate, the
influence (or association) of sex on the results of the study. Manuscripts reporting research
involving animals must include the protocol approval number of the Institutional Animal Care
and Use Committee (IACUC) or other standardized evidence that the animal care complied with
the “Guide for the Care and Use of Laboratory Animals” (www.grants.nih.gov/gran
ts/olaw/guide-
for-the-care-and-use-of-laboratory-animals.pdf).
AUTHORSHIP
Any person listed as a manuscript author should have made substantive intellectual contributions
to the study as established by the International Committee of Medical Journal Editors (ICMJE,
www.icmje.org
). All authors should meet each of the following conditions with regard to the
manuscript: (1) substantial contributions to conception and design, or acquisition of data, or
analysis and interpretation of data; (2) active role in drafting the article or revising it critically for
important intellectual content; (3) final approval of the version to be published; and (4)
accountability for the accuracy and integrity of the article. Duties of authors may be reviewed at
www.elsevier.com/about/policies/publishing-ethics.

To ensure appropriate credit for publication, authors are strongly encouraged to provide Open
Researcher and Contributor Identification (ORCID) numbers. This author-specific, cross-
platform, persistent digital identifier may be obtained free of charge at www.orcid.org
.
THE USE OF ARTIFICIAL INTELLIGENCE (AI)
JVIR encourages the use of artificial intelligence (AI) to improve grammar, spelling, and
readability of submissions, and is aligned with the recommendations of ICMJE, WAME, and CSE
.
Where authors use artificial intelligence (AI) and AI-assisted technologies in the writing process,
authors must:
1) Only use these technologies to improve readability and language, not to replace key
researcher tasks such as interpreting data or drawing scientific conclusions.
2) Apply the technology with human oversight and control, and carefully review and edit the
result, since AI can generate authoritative-sounding output that can be incorrect, incomplete
or biased.
3) Do not list AI and AI-assisted technologies as an author or co-author, or cite AI as an
author. Authorship implies responsibilities and tasks that can only be attributed to and
performed by humans.
4) Disclose in the manuscript the use of AI and AI-assisted technologies in the writing process
by following the instructions below. Please note that authors are ultimately responsible and
accountable for the contents of the work.
Disclosure instructions
Authors must disclose the use of AI and AI-assisted technologies in the writing process by
adding a statement at the end of their manuscript in a new section entitled ‘Declaration of AI
and AI-assisted technologies in the writing process’. Statement: During the preparation of
this work the author(s) used [NAME TOOL / SERVICE] in order to [REASON]. After using
this tool/service, the author(s) reviewed and edited the content as needed and take(s) full
responsibility for the content of the publication. This declaration does not apply to the use of
basic tools for checking grammar, spelling, etc. If there is nothing to disclose, there is no need
to add a statement.
SPONSORSHIP and FUNDING: SOURCES and ROLES
Authors are required during the submission process to identify who provided financial support for
the conduct of the research. If the manuscript describes a sponsored study, it must be made clear
in the submission what roles the sponsor played – see
www.councilscienceeditors.org/2-4-
sponsor-roles-and-responsibilities. If any writing assistance other than copy editing or legal
review was provided, the name of the person(s) and employer must be provided. The six-item
Study Sponsor Checklist must be completed for each sponsored study submission as part of the
Title page:
1. Was this study initiated by the sponsor?

2. Did the sponsor participate in the design of the study?
3. Was the sponsor involved in the collection, analysis, and/or interpretation of the data?
4. Did the sponsor participate in writing the first draft of the manuscript?
5. Did the sponsor provide any writing assistance, other than copy editing?
6. Was the sponsor involved in the decision to submit the manuscript for publication?
Please provide details for any questions answered “yes.”
Any agreements concerning confidentiality of the data between the sponsor and the authors or the
institutions named in the credit lines must be disclosed, and a copy of the study protocol must be
uploaded with the manuscript
. Any restriction of access to original data, or drafting or writing of
manuscripts by sponsoring entities without crediting and disclosing such authorship, is prohibited
(www.wame.org/ghost-writing-initiated-commercial-companies
).
Grant identifiers must be provided on the title page but not in the text, to protect the
confidentiality of the double-blind review.
CONFLICTS OF INTEREST
JVIR adheres to the policy on conflicts of interest of the ICMJE, which states, in part, that “to
prevent ambiguity, authors must state explicitly whether potential conflicts do or do not exist.”
Authors must state their disclosures on the title page of the manuscript. If there are no disclosures,
state “Conflicts of interest: none.” Each author of each manuscript will be required to complete an
ICMJE disclosure form at the time of submission (www.icmje.org/conflicts-of-interest/
), detailing
all relationships with any possible perceived relevance held within the 36 months before the date
of submission. Corresponding authors will be responsible for collecting these and uploading them
during the submission process. Authors in the USA are required to verify disclosed conflicts with
payments listed on the federal government Open Payments site:
openpaymentsdata.cms.gov/.
REDUNDANT (DUPLICATE) PUBLICATIONS, PIRACY, and PLAGIARISM
DEFINITIONS
A publication is considered to be redundant (duplicate) when it contains extensive and
unacknowledged verbatim or near-verbatim reproduction in print or electronic media of
previously published original or review articles. Duplication also extends to submissions
under consideration by another journal as well as to presentations and posting of results
in registries. Piracy is defined as unauthorized reproduction or use of ideas, data, or
methods from others without adequate permission or acknowledgement (CSE).
Plagiarism is a form of piracy involving duplication or close imitation of text, figures
and/or tables. The word “extensive” refers to substantial overlaps, understood as
duplication of the entire manuscript or of entire paragraphs or sections. The word
“unacknowledged” refers to unauthorized use of the same data in several publications,
that is, use “without appropriate justification, permission or cross-referencing” (COPE).
The reuse of “significant, identical, or nearly identical portions of own previously
published worked without citing the earlier publications or without citing the original
papers” (ISMTE) is considered to be “self-plagiarism” (CSE). Citing previously
published work does not in itself render extensive duplication acceptable.

Sources
International Committee of Medical Journal Editors (ICMJE) Guidelines. Uniform
Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for
Biomedical Publications. Available from: www.icmje.org
Committee on Publication Ethics (COPE). Code of conduct and best-practice guidelines
for journal editors; Dual publication; Suspected redundant publication in a submitted
manuscript (flowchart). Available from: www.publicationethics.org
Council of Science Editors (CSE). White paper on promoting integrity in scientific
journal publications. Available from: www.councilscienceeditors.org/
International Society of Managing and Technical Editors (ISMTE). Publishing Ethics
101: A Guide for the Editorial Office. Available from: www.ismte.org/
World Association of Medical Editors (WAME). Duplicate submissions. Available from:
www.wame.org/resources
Elsevier’s Policy: Multiple, duplicate, and concurrent publications. Available from:
www.elsevier.com/editors/perk/multiple-duplicate-concurrent-publication-simultaneous-
submission
In addition, Elsevier’s general sections on publishing ethics are available at
www.elsevier.com/about/policies/publishing-ethics
JVIR’S POSITION
Submission of material without citation of the self-same material information elsewhere
and without permission from the copyright holder is a violation of ethical publishing
norms. This principle extends to dual or multiple submissions (that is, submission of
material to a journal when the same material is already under consideration by
another/other journal[s]). This is especially true when there is “evidence that authors have
sought to hide redundancy, e.g., by changing title, authors’ order, or not referring to
previous papers” (COPE). JVIR prohibits these practices and is mandated to act upon
infractions. JVIR uses duplication detection software to help identify redundancy and
piracy. Authors are encouraged to use publicly available duplication detection software
prior to submission of manuscripts.
SPECIAL CASES
JVIR understands that duplicate publication is permissible under certain circumstances
(e.g., publication in a foreign language, for a completely different audience, or in a
special commemorative edition) as long as credit is given to the previous publication and
permission to reprint is granted by the copyright holder. If the author(s) considers
duplicate publication in the future, the editorial office should be notified. For more details
on special cases when duplicate publication is permitted, see Elsevier’s policy: Multiple
publications under Sources above. If a study has been previously presented as an abstract
at the SIR Annual Scientific Meeting or any other meeting where the proceedings and
abstracts were published, the meeting, year, abstract number, and title must be specified

in the title page. Content may overlap with abstracts, but text should not be copied and
pasted verbatim.
TEXT RECYCLING
1. Reuse of material from authors’ own published work, sometimes referred to as “text
recycling” but also sometimes considered “self-plagiarism,” must conform to standards
of publication ethics and copyright law, as described at textrecycling.org/
and
https://publicationethics.org/sites/default/files/Web_A29298_COPE_Text_Recycling.pdf.
2. Reuse of portions of a Materials and Methods section may be permissible, or may be
preferable for the purposes of clarity and consistency. For instance, if an interim analysis
is published on a prospective trial, the final analysis may benefit from reuse of the same
description of the study protocol.
3. The recycling of text must be disclosed upon submission of the article. Extensive
recycled text should be indicated by quotation marks and/or section indentation, with
appropriate reference citations.
4. Background information in the Introduction and Discussion, even if matching in
content to those of the preexisting publication, should be rewritten and rephrased.
5. Hypotheses, results, data analyses, conclusions, figures, and tables must not use
recycled text.
6. Any text recycling that may violate copyright law must be avoided. This includes reuse
of material by an author group that does not include all of the previous authors of the
original text, if copyright is held by the original author group. Reuse of material for
which the copyright is held by a publisher or society requires written permission for reuse
by the publisher or society.
7. Text previously published in JVIR as an SIR annual meeting abstract may be recycled
in most cases for article submissions to JVIR. Recycling of text from abstracts from non-
SIR meetings may require obtaining permission to reuse from the copyright holder.
Recycling of authors’ own text from non-copyrighted materials, such as grant proposals
and oral presentations, may be acceptable.
REPORTING STANDARDS
SIR REPORTING STANDARDS
In order to ensure consistency in reporting the results of clinical research, SIR has developed a
number of reporting standards documents that authors should follow when submitting
manuscripts for consideration. Adherence to relevant reporting standards will be taken into
account in the review process. Refer to www.jvir.org/content/reporting
.
STANDARDS BY STUDY TYPE
For a summary of study type reporting standards, see www.equator-network.org/
.
CONSORT STATEMENT
JVIR formally endorses the CONSORT (Consolidated Standards of Reporting Trials)
Statement. The CONSORT Statement contains criteria developed to improve the quality
of published reports of randomized clinical trials. The 2010 criteria consist of a 25-item
checklist that pertains to the various sections of a report of a clinical trial (Title, Abstract,
Introduction, Materials and Methods, Results, and Discussion). The 2017 Update and
Extension for Nonpharmacologic Trials and Abstracts added 5 new items, which may be
pertinent to articles submitted to JVIR. Authors of randomized clinical trials are required
to upload the CONSORT criteria and checklist when submitting a manuscript. The
CONSORT Flow Diagram should be submitted as Figure 1. The checklist must be
submitted, but will not be published. For more information on the CONSORT Statement,
please visit www.consort-statement.org
.
STROBE STATEMENT
JVIR supports the STROBE (STrengthening the Reporting of OBservational studies in
Epidemiology) Statement. Although JVIR does not exclusively publish epidemiology
articles, the guidelines are generalized to all observational studies and, like CONSORT
criteria, consist of a 22-item checklist to aid authors to produce comprehensive
manuscripts. The current 2007 Version 4 Checklists are available for cohort studies, case-
control studies, and cross-sectional studies, at www.strobe-statement.org
.
PRISMA STATEMENT
JVIR supports the PRISMA (Preferred Reporting Items for Systematic reviews and
Meta-Analyses) Statement. Like CONSORT and STROBE, the PRISMA statement
includes a 27-item checklist of information that must be included in a comprehensive
journal article. In addition, like CONSORT, PRISMA incorporates a flow diagram that
should be completed and submitted as Figure 1, describing the study selection process for
the submitted systematic review or meta-analysis. Details and downloadable checklist
and flow diagram are available at www.prisma-statement.org
.
ARRIVE STATEMENT
JVIR supports the ARRIVE (Animal Research: Reporting of In Vivo Experiments)
guidelines. Like other statements, the ARRIVE guidelines support a 10-item checklist of
information that must be included in journal publications to improve the reporting of
animal research. Details and downloadable checklist are available at
www.arriveguidelines.org.
STATISTICAL METHODS AND STANDARDS
JVIR advises authors without formal biomedical statistics training to consult professional
statisticians prior to performance of statistical tests to be reported in the manuscript. Statistical
details should be reported according to standards, as described in www.ICMJE.org
or SAMPL
(www.equator-network.org) guidelines. In situations where the contributions of a statistician are
fundamental to the manuscript, the statistician should be listed as a co-author. Additional details

are available at www.elsevier.com/__data/promis_misc/jvir-guidelines-for-statistical-
methods.pdf.
OPEN ACCESS
Information on Open Access options may be found on the JVIR information page at
www.elsevier.com/journals/jvir-journal-of-vascular-and-interventional-radiology/1051-
0443/open-access-options.
Aside from Open Access options, JVIR does not levy article processing charges or page charges.
Invoices received by authors demanding payment should be considered fraudulent, and should be
reported to jvir@sirweb.org and the Elsevier Research Support team. See
www.elsevier.com/connect/authors-update/seven-top-tips-on-stopping-apc-scams.
FUNDING BODY AGREEMENTS AND POLICIES
Elsevier has established a number of agreements with funding bodies which allow authors to
comply with their funder's open access policies. Some funding bodies will reimburse the author
for the gold open access publication fee. Details of existing agreements are available online
(www.elsevier.com/about/open-science/open-access/agreements
).
AUTHORS FOR WHOM ENGLISH IS NOT THE PRIMARY LANGUAGE
JVIR publishes manuscripts only in English, using the American style of spelling and decimal
points. For authors for whom English is a secondary language, language editing services are
available through commercial services, including the publisher, Elsevier. JVIR does not endorse
or guarantee their work. Additional details on JVIR writing style are available at
www.elsevier.com/__data/promis_misc/jvir_manprep.pdf
. Submitted manuscripts in which the
English language is not correctable with minor copy editing will be declined and returned to the
authors for professional editing prior to resubmission. If authors choose to use an artificial
intelligence program for language editing, both the original and AI-edited versions of the
manuscript should be submitted.
MANUSCRIPT PREPARATION
DOCUMENT TECHNICAL SPECIFICATIONS
JVIR publishes several types of articles, each of which has a distinct format. The preferred word
processing program is Microsoft Word.
1. Manuscripts must be written with 12-point font, double-spaced throughout (including tables,
references, and figure legends), and have at least 1-inch (3-cm) margins.
2. The text should be ragged right (no right justification). Embedded instructions (e.g., italics,
underlines, boldface) should be kept to a minimum. Do not use coding for centering.
3. Insert only one space after punctuation marks.
4. Sequential page numbering should begin with the abstract as page 1.
5. Line numbering should start with the abstract and be continuous (without starting over at line
1 on each page) through the Figure Legends and Supplementary materials.
6. Please avoid first person verbiage (I, we, our, etc.).

7. Please avoid claims of primacy (“first ever reported”).
8. Each article should include at least one image, so that it may be used as a thumbnail.
9. A cover letter addressed to the editor is optional, and if included, is limited to 250 words.
Additional details on JVIR writing style and format are available at
www.elsevier.com/__data/promis_misc/jvir_manprep.pdf
.
BLINDING OF MANUSCRIPT
JVIR adheres to a double-blind review process, whereby the identities of the authors are kept
confidential from the reviewers and vice versa. To ensure blinded peer-review, no direct
references to the author(s), institution(s) of origin, or previous work/publications should be made
anywhere in the abstract, text, figure legends, tables, footnotes, list of references, appendixes, or
file names. Authors should avoid wording such as: “In a previous article (3), we reported…”,
“Procedures were performed by two investigators (A.B.C., X.Y.Z.)…”, “Patients enrolled at
University Hospital, a tertiary center in Capital City, State…”,“Authors acknowledge
proofreading provided by John Smith…”, “Research was supported by NIH R01…”, “Trial is
registered on clinicaltrials.gov
number …” Relevant identifying information may be included in
the Title Page, and authors will be able to unblind the blinded information after the article is
accepted for publication.
KEY WORDS
JVIR does not publish key words, but authors may submit a list of key words to improve
discoverability after publication. Authors are encouraged to use Medical Subject Headings
(MeSH), which are listed at www.nlm.nih.gov/mesh/meshhome.html
, and to include these terms
in the article title and abstract to improve discoverability.
TYPES OF SUBMISSIONS
CLINICAL STUDY AND LABORATORY INVESTIGATION
Clinical Studies (involving human subjects) and Laboratory Investigations are full-length,
original research documents, with higher requirements for level of evidence and expected impact.
Length is limited to 3500 words of body text, not including references, tables, table legends, or
figure legends. References are limited to a maximum of 35. Authors are encouraged to make
judicious use of supplemental appendices, tables, and figures (published as online supplements)
to ensure compliance with word count and figure limits. The order of sections is: Title Page,
Abstract, Text, References, Tables, Figure Legends, Figures, Supplementary materials, ICMJE
disclosures.
ABSTRACT
The abstract for original clinical and laboratory investigations should be no longer than 250
words and should be formatted into discrete sections titled Purpose, Materials and Methods,
Results, and Conclusion. The abstract should summarize all of the main aspects of the study. The
Purpose statement should be a single hypothesis-driven sentence, and background information is
not necessary. Actual data with statistics should be included in the Results. The Conclusion
should be limited to what was drawn directly from the study. Note that the Conclusion will be
used as a summary statement of the work in the printed Table of Contents.
TEXT
• Introduction: Provide a brief summary (usually 250–350 words) of background material to set
the stage for the article. This section should end with a succinct statement of the hypothesis-
driven purpose of the study.
• Materials and Methods: Describe the nature of the subjects, methods of selection, materials
(including generic description [model name; manufacturer’s name, and headquarter location city
and state or country if not the USA]), and all procedures. The number of participants and
demographics of study group(s) (such as sex distribution, mean age, underlying medical
problems) should be included in this section. References should be made to established methods
that have been published. New or substantially modified methods should be described, supported
with rationale, and critically evaluated for real and potential limitations. This section should
conclude with a description of all statistical methods used to analyze the data, with references and
names of computer software packages.
• Results: Report of data and observations should be in logical sequence in the text, tables, and
figures, reflecting the sequence in the Materials and Methods section. Tables and figures should
be called out in the text. Data given in tables should not be repeated in the text. Complex reports
may require subheadings in this section. Supporting but non-essential data may be submitted as
Supplemental Materials for inclusion in the electronic version only.
• Discussion: A brief summary of the relevant new knowledge gained should be followed by
placing this knowledge into perspective. Consider only new and important aspects of the study
and conclusions that can be drawn directly from the data. Include implications of findings, and
relate observations to other relevant studies. Include a separate paragraph that outlines the
limitations of the study. Avoid claiming primacy, alluding to work that has not been completed,
or making unqualified statements not supported by the data. Avoid gratuitous calls for
randomized trials. Clinical practice recommendations should be made when appropriate. The last
paragraph is typically 1-3 sentences summarizing the article. Length is typically fewer than 1000
words.
REFERENCES
In Text Citations: Number the references in the order in which they appear in the text (including
references in tables at the site where they are mentioned in the text). Reference numbers appear in
line within parentheses (not bracketed, not superscripted). Make sure the number used for the
reference cited in the text matches the number of the respective reference in the references list.
Note: Unpublished data are not cited in the reference list but cited parenthetically in the text, and
are generally discouraged.
References List | Reference Style: References must be current and relevant. JVIR no longer
requires authors to use a strict style for reference formatting at submission. References can be in
any style or format as long as the style is consistent. However, each reference must include the
digital object identifier(DOI). JVIR’s reference style will be applied to the accepted article by
Elsevier at the proof stage. Note that missing data will be highlighted at proof stage for the author
to correct.
RESEARCH HIGHLIGHTS

Authors of Clinical Studies, Laboratory Investigations, Evidence-Based and Narrative Reviews,
and Brief Reports are required to submit a short, bulleted list of Research Highlights. These
highlights should consist of 3–5 concise points for full length articles, limited to 100 words, and
1-3 points for Brief Reports, limited to 50 words, conveying core findings and conclusions,
uploaded as a separate file. The Editors may revise the highlights or rewrite them, or add their
own perspectives on the value of the research. Proposed research highlights should be submitted
as a separate editable file as part of the online manuscript submission, using “Research
Highlights” in the file name.
BRIEF REPORT
Brief Reports may be either clinical or nonclinical, more exploratory or preliminary or lower
level of evidence, and narrower in scope than Clinical Study and Laboratory Investigation
manuscripts. The length is limited to 1800 words of body text. References are limited to a
maximum of 15, and figures are limited to 8 figure parts. The manuscript components are
identical to those of Clinical Study and Laboratory Investigation manuscripts, and the order of
sections is: Title Page, Abstract, Text, References, Tables, Figure Legends, Figures,
Supplementary materials, ICMJE disclosures. However, for brief reports, the abstract is a short
(maximum 150 words) unstructured paragraph.
LETTER TO THE EDITOR (including CASE REPORT)
Letters to the Editor may offer commentary on any material already published in JVIR. Letters
that relate to a published article will be published pending response from the original article’s
author(s). Letters to the Editor may also be used to convey limited new material of general
interest to the interventional radiology community. In general, individual case reports or small
case series should be submitted as Letters to the Editor. Length is limited to 800 words, plus up to
4 references. Figures are limited to 6 figure parts. Author list should be no more than 6
individuals. The order of sections is: Title Page, Letter, References, Tables, Figure Legends,
Figures, ICMJE disclosures.
EVIDENCE-BASED REVIEW AND NARRATIVE REVIEW
JVIR will review unsolicited Evidence-Based Review articles, which are systematic reviews and
meta-analyses. Authors are highly encouraged to register systematic reviews with PROSPERO
(www.crd.york.ac.uk/prospero/
) prior to starting work on the article to avoid potential
duplication. Length is limited to 5000 words of body text, plus up to 75 references. The order of
sections is: Title Page, Abstract, Text, References, Tables, Figure Legends, Figures,
Supplementary materials, ICMJE disclosures. In addition, Narrative Review Articles may be
invited by the Editor but are still subject to peer review and are not guaranteed acceptance.
Authors may consult the Editor with proposals prior to preparation and submission of unsolicited
Narrative Review Articles. The sections and length limits are identical to Evidence-Based
Reviews.
RESEARCH IN TRANSLATION
Research in Translation articles introduce innovative basic or preclinical concepts that may be
advancing towards clinical care in interventional radiology. Articles should focus on relevance
and the path to clinical application. A multidisciplinary author group is highly recommended.
Authors may consult the Editor with proposals prior to preparation and submission of unsolicited
translation articles. Text is limited to 1800 words of body text. The order of sections is: Title
Page, Introduction, Concept, Relevance, Translation, References (maximum 20, with judicious
use of a suggested reading list for online publication), Table, Figure Legends, Figures,
Supplementary materials, ICMJE disclosures.
STUDY PROTOCOL DESIGN
Study Protocol Design articles outline prospective clinical trials, studies, and registries with
finalized designs that are starting up, actively enrolling, or approved to enroll. Funded studies,
such as those supported by the SIR Foundation, NIH, NSF, PCORI, DoD, etc. with relevance in
the field of interventional radiology are especially encouraged to publish the study protocol
design in JVIR. Study Protocol Design articles are designed to increase awareness, referrals, and
enrollment in clinical trials, encourage hypothesis-based prospective research, facilitate feedback
to authors prior to completion of studies, allow researchers an early record of development of
original ideas and methodologies, and reduce redundancy of research efforts.
All human subject trials must include a registration number with clinicaltrials.gov
or equivalent
system. The length is limited to 700 words of body text, and should include a succinct (<200
word) Introduction describing the background and purpose of the study. JVIR suggests using the
SPIRIT reporting guidelines to create a bulleted list of essential information, including sponsor
information, trial design (including randomization process if applicable), study population,
interventions, follow-up, sample size calculation, and study endpoints/outcome measures. No
abstract is required. References are limited to a maximum of 8. Tables are limited to 1 summary
of eligibility (inclusion and exclusion) criteria. Figures are limited to 1 diagram of the trial
schema or timeline. Additional information may be supplied as Supplementary materials for
online only publication. If the article describes an actively enrolling clinical trial, the contact
information for the study coordinator should be included. The order of sections is: Title Page,
Introductory text, Bulleted list, References, Table, Figure Legend, Figure, Supplementary
materials, ICMJE disclosures.
LESSONS IN IR (Morbidity & Mortality)
Lessons in IR (M&M) articles describe a single clinical case in which an adverse event occurred
during interventional radiological care. Cases should have broad educational appeal (including to
students and trainees), and thus should be instructive rather than extreme or exceptional. They
may portray procedural or post-procedural adverse events or predicaments, mitigative actions,
and outcomes. All aspects must be de-identified and compliant with the Health Insurance
Portability and Accountability Act (HIPAA), approved or waived by an institutional review
board, and free of current and past medicolegal litigation, arbitration, and patient complaints. All
Lessons in IR (M&M) articles will have a legal disclaimer attached for publication. Text is
limited to 500 words of Case Description body text and Discussion section, plus a maximum of 3
references. The Discussion should consist of 3 subsections entitled “Preparation,” consisting of
pre-procedural appraisal and recognition of unique risk factors as well as a possible plan of action
to mitigate the adverse event should it occur; “Avoidance,” consisting of procedural best practices
to preclude the adverse event; and “Management,” consisting of procedural maneuvers or post-
procedural care to mitigate the event. Each subsection should include no more than 2-4 relevant
bullet points. Figure descriptions should be included in the text rather than in figure legends, and
are limited to 6 figure parts. Each case should be assigned a Society of Interventional Radiology
(SIR) adverse event severity assessment (see Baerlocher et al.,
www.jvir.org/article/S1051-
0443(22)01252-0/fulltext). The order of sections is: Title Page, Case Description, Discussion,
SIR adverse event severity assignment, References, Figures, ICMJE disclosures.
EXTREME IR
Extreme IR articles describe a single clinical case in which extraordinary measures were
required. Cases may portray severe pathology, unexpected clinical situations, or unanticipated
procedural dilemmas demanding creative solutions. Text is limited to 350 words including case
description body text and figure legends, and should include no references to allow for high
quality, instructive figures or illustrations, limited to 6 figure parts. The order of sections is: Title
Page, Text, Figure Legends, Figures, ICMJE disclosures.
IMAGES IN IR
Images in IR articles consist of 1–4 images demonstrating a unique anatomic finding, an unusual
diagnosis, or otherwise striking image encountered in clinical interventional radiologic practice.
Text is limited to 150 words of figure legends and should include no body text and no references.
The order of sections is: Title Page, Figure Captions, Figures, ICMJE disclosures.
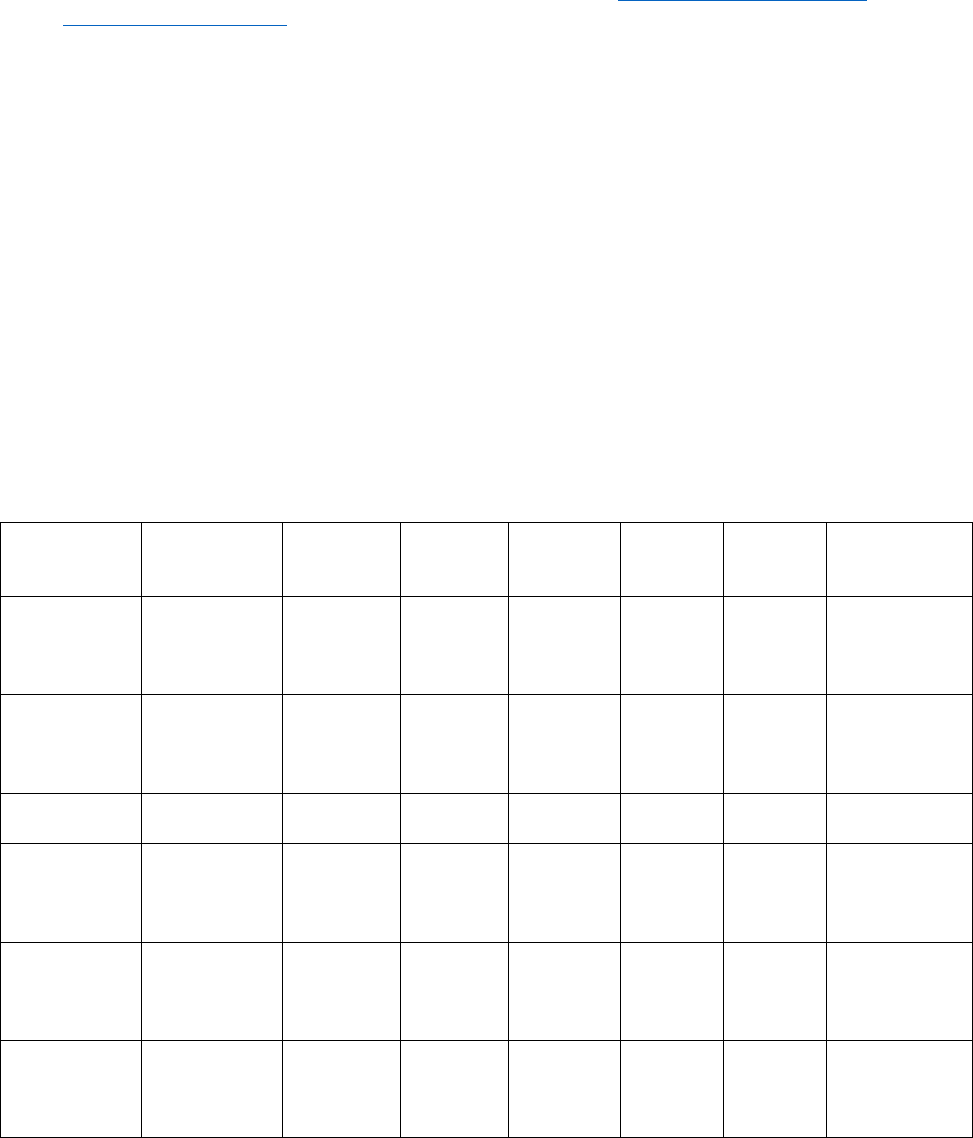
Manuscript
type
Abstract
Research
Highlights
Body text
word limit
References
maximum
Tables
maximum
Figure
part
maximum
Supplementary
Materials
Full length
(Clinical Study,
Laboratory
Investigation)
Structured,
250-word
limit
3–5
bulleted
points, 100
word limit
3500 35 6 12 No set limit
Brief Report
Unstructured,
150-word
limit
1-3
bulleted
points, 50
word limit
1800 15 4 8 No set limit
Letter to the
Editor
NA NA 800 4 2 6 No set limit
Evidence-
based
(Systematic)
Review
Structured,
250-word
limit
3–5
bulleted
points, 100
word limit
5000 75 10 12 No set limit
Narrative
Review
Unstructured,
150-word
limit
3–5
bulleted
points, 100
word limit
5000 75 10 12 No set limit
Research in
Translation
Unstructured,
150-word
limit
NA 1800 20 1 8 No set limit
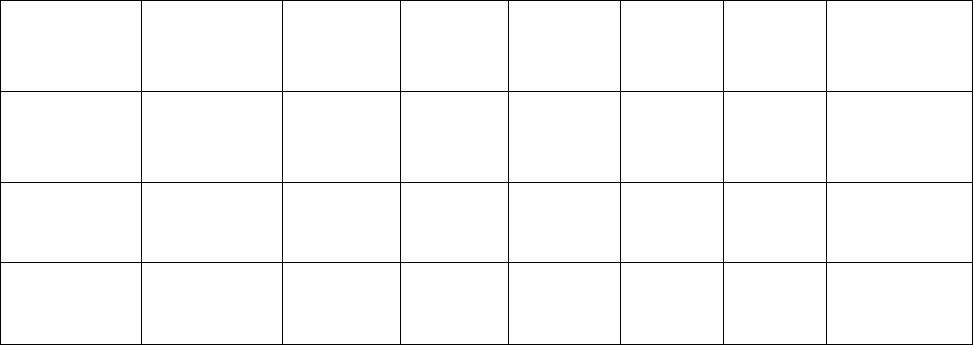
Study Protocol
Design
NA NA 700 8 1 1 No set limit
Lessons in IR:
M&M
NA NA 500 4 0 6 No set limit
Extreme IR NA NA
350 (body
text +
legends)
0 0 6 No set limit
Images in IR NA NA
150
(captions
only)
0 0 4 No set limit
IN MEMORIAM
In Memoriam pieces are dedicated to recently deceased personalities of the IR community. Text
is limited to 650 words, and a photographic portrait of the memorialized person should be
included.
EDITORIAL, COMMENTARY, IR HISTORY, BOOK REVIEW, PERSPECTIVE
Editorial, Commentary, and IR History articles are typically invited by the Editor. Book
Reviews are invited by the Book Review Editor. Authors may also contact the Editor to propose a
Perspective, History article, or Book Review. Specific instructions are provided at the time of
invitation. JVIR will not accept an unsolicited Commentary, but authors are encouraged to
submit a Letter to the Editor to comment on material already published in JVIR.
SUPPLEMENTS, CONFERENCE PROCEEDINGS, MEETING ABSTRACTS
Scientific abstracts (excluding educational posters) presented at the Annual Scientific Meeting of
SIR are published as a separate journal supplement. Under special circumstances, abstracts and
conference proceedings representing peer-reviewed research from other scientific meetings may
be published as supplemental material with prior agreement between the meeting chairs and the
Editor.
SIR AND SIR FOUNDATION DOCUMENTS
Official documents originating from SIR or SIR Foundation will be given high profile and
expanded access as JVIR publications. These include Clinical Practice Guidelines, Position
Statements, Research Reporting Standards, and Research Consensus Panel Proceedings.
Special manuscript preparation instructions may be obtained from SIR or SIR Foundation.
ONLINE-ONLY PUBLICATION
SUPPLEMENTARY MATERIALS
To comply with printed word limits, JVIR will allow or encourage publication of additional
tables, figures, or text (e.g., methodology details, comprehensive data, complementary images,
etc.) in the electronic version of the published manuscript. This material will not be included in
the print version but a reference to its availability online will be present in the print version.
Supplementary material must meet strict criteria to be included in the electronic version and must
not be redundant or irrelevant data. Online-only supplementary material must be marked clearly
in the submitted manuscript.
1. Number online-only materials separately, by adding the prefix E (e.g., Fig. E1, Fig. E2).
2. Number the figures and tables sequentially in the order in which they are called out in the text.
3. In-text citations must match the figure/table numbers for print and for online-only E-
publication. Citations for print and online-only materials may be interspersed (e.g., Fig. 1, Fig. 2,
Fig. E1, Fig. 3).
DATA REPOSITORY
In alignment with the ICMJE and other organizations, JVIR supports responsible data-sharing for
interventional clinical studies. This practice supports transparency, results verification, and secondary
analysis (systematic review and meta-analysis) generation. JVIR encourages authors to upload a
manuscript’s source data and to cite underlying or relevant datasets in manuscripts by citing them in the
text and including a data reference in the reference list. Data references should include the following
elements: author name(s), dataset title, data repository site, version (where available), year, and global
persistent identifier. Add [dataset] immediately before the reference so that it can be properly identified as
a data reference. The [dataset] identifier will not appear in the published article.
Mendeley Data is a free-to-use open research data repository designed for this purpose and owned by
JVIR’s publisher Elsevier. To make a manuscript’s data available, authors may create a dataset at
Mendeley Data at data.mendeley.com/
and publish it (under embargo if desired). If authors use Mendeley
for data deposit, Elsevier will place links between the article and the dataset, making the data easily
accessible to readers. Open source code may also be posted on Github (
github.com).
MANUSCRIPT SUBMISSION PROCESS
GENERAL INFORMATION
All new manuscripts must be submitted through the JVIR online submission site at
www.editorialmanager.com/JVIR
. Authors are required to upload the title page, text, and tables
as Microsoft Word .docx files, and separate figures in electronic form not embedded in the Word
file or PDF. Manuscript word count (including main text and references) should be listed on the
title page, and the text must have page numbers printed at the bottom of each page starting with
the abstract page as page 1. If the study was sponsored, the 6-item study sponsor checklist must
be completed and included on the Title page.

NEW SUBMISSIONS
• An optional cover letter may be uploaded as a separate file. Cover letters are seen only by the
editors and should only provide information not included in the manuscript text, such as
information on the roles played by the funders of externally sponsored trials, and whether any
aspects have been presented or published, or posted as a preprint. Authors may nominate
appropriate objective, nonconflicted, expert reviewers.
• Title page
• Research highlights (for full length articles only)
• Blinded manuscript
• Tables
• Figures
• Supplementary material
• ICMJE disclosures
REVISIONS
• Optional cover letter
• Title page
• Research highlights (for full length articles only)
• Point-by-Point Response to Review as a separate document using the Word table template
provided at www.jvir.org/content/authorinformation
. This document should outline how authors
dealt with each of the points raised by the editors and reviewers. Authors need not agree with all
of the suggestions or criticisms but must explain the authors’ position on every point. Revisions
of the manuscript (if performed) must be specified for each comment. Replies to comments will
not be published—only the revisions to the manuscript.
• Clean, blinded manuscript that incorporates any changes made during the revision process
• Manuscript with tracked changes. Set the word processing program track changes options to
color only/blue for inserted text and to strikethrough/red for deleted text.
• Tables
• Figures
• Supplementary material
• ICMJE disclosures
FILE NAMES
Files should be labeled with descriptive file names (e.g., Coverletter.docx, Manuscript.docx,
Revised_manuscript.docx, Table3.docx, Fig1a.tif). Upload text, tables, and figures as separate
files. Do not embed figures or tables into the text document, and do not upload any of the
materials as a PDF.
TECHNICAL SPECIFICATIONS FOR TABLES AND FIGURES
TABLES

• Use Microsoft Word’s Table feature. Do not construct tables using tabs. Do not use Excel or
comparable spreadsheets.
• Do not use vertical/horizontal lines or shading.
• Table title and table legend (if one is necessary) should be included in the same file.
• Tables must be uploaded as individual files, one for each table, and include the table number in
the file name (e.g., Table3.docx). Do not embed tables into the text file.
• Do not submit single-column tables. A single column table should be converted into a list or
incorporated into the text.
FIGURES (IMAGES)
Graphics software such as Photoshop, Illustrator, BioRender, Canva, or InkScape should be used
to create camera-ready art. Submit figure
images electronically as individual files saved in TIF or
EPS file format. Multiple panel figures (e.g., Fig. 1a, 1b, 1c, 1d) must be submitted one panel
image per file and not as composite images. Figures submitted embedded in the text file or in
presentation software such as PowerPoint, CorelDraw, or Keynote will be rejected. Original art
must be prepared and submitted at the proper resolution and size. Editing of images for clarity
(cropping, rotation, brightness and contrast, color balance, elimination of artifacts) is encouraged,
but manipulation resulting in misrepresentation, removal of legitimate, or introduction of
fabricated data is prohibited. If images required extensive alteration, both the source image and
the edited image must be submitted.
Step-by-step instructions for art preparation are available at
www.elsevier.com/artworkinstructions
. Graphics services are also available through the publisher
at www.webshop.elsevier.com/illustration-services/. Manuscripts may move into peer review
even if the figures do not meet production standards; however, figures of adequate quality are
required for publication in JVIR, and failure to provide adequate figures will delay or block
publication.
COLOR FIGURE POLICY
JVIR publishes in full color. However, reproduction of articles and figures by users and readers
may not be in color, so color figures must be prepared so that conversion to grayscale does not
compromise their abilities to convey meaning. Color figures should be prepared to be accessible
to readers with color vision deficiency; instructions are available at
www.elsevier.com/authors/policies-and-guidelines/artwork-and-media-instructions
.
TECHNICAL SPECIFICATIONS FOR RADIOGRAPHS, PHOTOGRAPHS, SCANNED IMAGES, AND
HALFTONES
(DIGITAL IMAGES CHARACTERIZED BY SHADING OR GRADIENTS)
Basic parameters
File Type: TIFF
Resolution: 300 dpi
Color mode: grayscale or RGB
Dimensions (inches): minimum 3.0” (smaller dimension)
File storage size (approximate)
Grayscale: 1–5 MB
RGB: 4–20 MB
TECHNICAL SPECIFICATIONS FOR LINE ART,
DIAGRAMS, DRAWINGS, AND GRAPHS (DIGITAL
LINE-DRAWN ILLUSTRATIONS WITHOUT GRADIENTS)
Basic parameters
File Type: TIFF or EPS
Resolution: Minimum 1000 dpi
Color mode: grayscale or RGB
Dimensions (inches): minimum 3.0” (smaller dimension)
File storage size (approximate)
Grayscale: 8–40 MB
RGB: 30–50 MB (not recommended; for largest file sizes, preparation as vector format or
conversion from raster to vector is preferred)
SIZE, RESOLUTION, LABELS, ARROWS
Figures should be prepared at the expected size of final printing, which is a maximum of
full-page width (7.5”) and a minimum of one column (3.5”). Most images are appropriate
for single-column size (3.5”). Unusually large and complex images may require full page
width, while multiple panel figures with small and simple panels may be fit into a row as
many as six images across one page. Even for multiple panel figures with expected small
panels, each panel should be prepared and submitted individually at 3.5” width. Figures
must be composed at full resolution from source data. Low resolution images that are
upscaled to higher resolution that remain pixelated and/or with compression artifacts are
not acceptable. Smartphone photographs of a computer monitor are definitely not
acceptable.
Labels such as figure part letters (a, b, c), arrows and arrowheads, asterisks, and axis
labels must be large and contrasted enough to be legible after potential minification in the
production process if an image is submitted larger than final print size and must be
reduced in size for publication. Figure part lettering should be in the lower left corner,
lower case in
Arial Bold font, black or white depending on background, and at least 12
pt size in final printed size. Other labels, including asterisks and other symbols on
radiographs, axis labels, graph symbols, and graph symbol keys should be at least 8 pt
final printed size. Arrows may be any color, preferably black or white for reproduction
quality, at least 3 pt (12 pixels) shaft weight, and at least 300% in arrowhead width and
length. Graph axes and other line work should be at least 1 pt weight. All
photomicrographs must include a scale bar.
VIDEO FIGURES
Video figures for online electronic publication: JVIR will accept relevant video clips with
accepted manuscripts for viewing in the online version. A representative thumbnail still image
from the video clip should be submitted to embed in the online publication as a visual link to the
video file. For video articles or video figures, authors are encouraged to utilize video editing
software such as Premiere Pro, Final Cut Pro, iMovie, or Windows Movie Maker.

TECHNICAL SPECIFICATIONS FOR VIDEOS
File format: MP4 (max target 720p), MOV, MPEG-1, MPEG-2, or AVI
Frame rate: 15 frames/second minimum
Video codec: H.264 (+AAC)
Video bit rate: 750 kbps preferred, 260 kbps minimum
Frame size: 492 x 276
Duration: 5 minutes maximum
File size: 150 MB maximum
VIDEO ARTICLES
Video Articles are no longer published by JVIR. Instructional videos may be appropriate for the
SIRnow video library, such as in the Early Career Section Channel. Please contact
education@sirweb.org
for information and submissions.
VISUAL ABSTRACTS
Full length Clinical Studies or Laboratory Investigations or Evidence-based Reviews may have a
Visual Abstract (graphical abstract, visual synopsis) composed by JVIR, in which the research
article is summarized diagrammatically. Authors are invited to submit drafts based on the format
of previously published JVIR Graphical Abstracts. Graphical Abstracts should be a standard 16:9
aspect ratio with 1920 x 1080 pixels at 300 dpi, minimum font size 14 pt, Gotham Narrow font,
preferring icons rather than illustrations, submitted in TIFF, PNG, or EPS format.
PROOFS
Authors’ pre-proof PDF version will be posted online at www.jvir.org
and listed on PubMed
upon final acceptance. Corresponding authors will receive an e-mail with a link to the online
proofing system, allowing annotation and correction of proofs online for final print and electronic
publication. The environment is similar to MS Word: in addition to editing text, authors can also
comment on figures/tables and answer questions from the Copy Editor. Web-based proofing
provides a faster and less error-prone process by allowing authors to directly type corrections,
eliminating the potential introduction of errors. In order to publish articles quickly and accurately
in JVIR, authors may use this proofing system only for checking the typesetting and editing for
completeness and correctness of the text, tables and figures. Significant changes to the article as
accepted for publication will only be considered at this stage with special permission from the
Editor. All corrections must be returned in one communication only. Please check carefully
before replying, since this is the only opportunity to make corrections. Proofreading is solely the
authors’ responsibility.
